The only naturally occurring isotope is the stable beryllium-9, although 11 other synthetic isotopes are known. Their half-lives range from 1.5 million years (for beryllium-10, which undergoes beta decay) to 6.7 × 10 −17 second for beryllium-8 (which decays by two- proton emission). Beryllium atom is alkaline earth metal atom with atomic number 4. It has a role as a carcinogenic agent, an adjuvant and an epitope. It is an alkaline earth metal atom and a metal allergen.
[Bohr Model of Beryllium] Neon Atom Model, Atom Model Project, Bohr Model. Visit [Bohr Model of Helium] Bohr Model, Homeschooling, Homeschool.
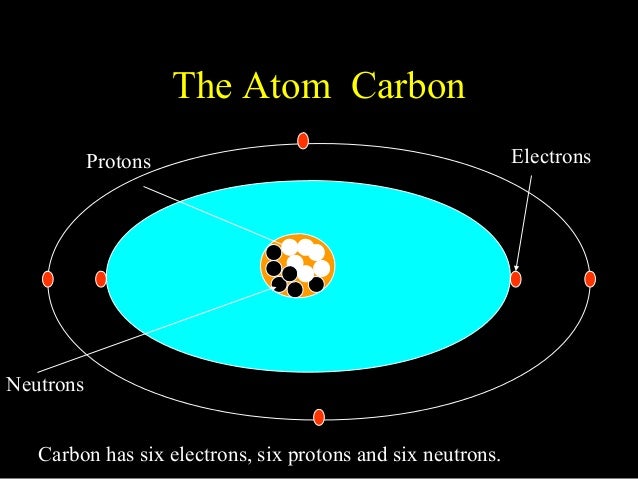
1 Draw a Bohr Model of Beryllium Draw a Bohr Model of Chlorine Activity Warm Up. Calculate the number of protons, neutrons & electrons for the following. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun.
In the Bohr model, electrons are. Atomic physics Bohr model of the atom.
by crator- Bohr And Quantum Mechanical Model of Atoms Bohr model and the quantum mechanical model. This means the beryllium atom has four protons and four electrons.
The number Creating a 3D model provides a child with a visual representation of a beryllium atom. Paint four How to Make a 3-D Bohr Model.
Copyright.The phosphorus bohr model has 3 shells because it has 15protons and 16 neutrons. ( is about 31, so 15protons = 16 neutrons the number of electrons is the same number of protons in an. Bohr Model Diagrams.
Use the information provided for each element to draw Bohr Model diagrams. Label how many of each there are in the nucleus (e.g.
He: 2p, 2n). Then, draw the individual electrons on the appropriate energy levels (keep in mind the maximum number of electrons allowed on each level – 2, 8, 8, etc.
1. Beryllium – P.
Properties Of Beryllium
E. N 2. A Bohr diagram is a simplified visual representation of an atom that was developed by Danish physicist Niels Bohr in The diagram depicts the atom as a positively charged nucleus surrounded by electrons that travel in circular orbits about the nucleus in discrete energy levels.
It's easier to understand electron configuration and valence if you can actually see the electrons surrounding atoms. For that, we have electron shell diagrams.
Here are electron shell atom diagrams for the elements, ordered by increasing atomic number. For each electron shell atom diagram, the element symbol is listed in the nucleus.
Facts Date of Discovery: Discoverer: Fredrich Wohler Name Origin: From the mineral beryl Uses: spacecraft, missiles, aircraft Obtained From: beryl, chrysoberyl Related Links I currently do not know of any links for Beryllium. If you do, please let me know MLA Format for Citing This Page.How to Make a 3D Beryllium Atom | SciencingWhat is the Bohr-Rutherford diagram of the beryllium atom
We elaborate the uses of Beryllium and atomic properties with characteristics. Beryllium is a metallic gray-white chemical element with atomic number 4. Its symbol is Be and belongs to the group of alkaline earth metals and its usual state in nature is solid. Beryllium is located at position 4 on the periodic table.
You Can Visit Our Managed: Periodic Table Main Page
On this page you can discover the chemical properties of beryllium and information about beryllium and other elements on the periodic table such as magnesium, lithium, boron or hydrogen. You will also learn what beryllium is for and you will learn what its uses are through its properties associated with beryllium such as its atomic number or the usual state in which beryllium can be found.
You will see qualities of beryllium such as its melting and boiling point, its magnetic properties or what its chemical symbol is. In addition, here you will find information about its atomic properties such as the distribution of electrons in beryllium atoms and other properties.
For some elements, some of this information is unknown. In these cases we show the properties attributed to them.
Beryllium properties
The alkaline earth metals, among which is beryllium, have properties among which is being soft, colored and having a low density. Elements like beryllium have low ionization energy. All alkaline earth metals form ionic compounds except for beryllium.
The state of beryllium in its natural form is solid (diamagnetic). Beryllium is a white-gray metallic chemical element and belongs to the group of alkaline earth metals. The atomic number of beryllium is 4. The chemical symbol for beryllium is Be. The melting point of beryllium is 1551.15 degrees Kelvin or 1279 degrees Celsius or degrees Celsius. The boiling point of beryllium is 3,243.15 Kelvin or 2,971 degrees Celsius or degrees Celsius.
Uses of beryllium
Beryllium is a light metal that has a high melting point and resists corrosion by concentrated nitric acid. If you have ever wondered what beryllium is for , here is a list of its possible uses:
- Probably the most important use of beryllium is in radiation windows for X-ray tubes. Beryllium is ideal for this use as it has very low X-ray absorption.
- Beryllium is used in the pipes of many high-energy particle physics collision experiments (such as the Large Hadron Collider). The stiffness of the metal allows to create a powerful vacuum.
- Beryllium is used as a lightweight component of military equipment and in the aerospace industry. It is used in high-speed aircraft, missiles, space vehicles, and communications satellites.
- It is one of the components of metal springs, non-sparking tools and electrical contacts.
- Naval personnel use beryllium tools when working with or near naval mines. Beryllium is a non-magnetic material and most naval mines detonate when they come in contact with something magnetic.
- Beryllium is used in the design of nuclear weapons. It is used as the outer layer of the well in the primary stage. It is an excellent inducer for implosion and is very good at reflecting neutrons.
- The low weight and high rigidity of beryllium make it perfect for use in high frequency speakers.
- Beryllium oxide is an excellent conductor of heat. For this reason, it is used in telecommunications by adding an insulating base plate of this material in high power transistors in radio frequency transmitters.
- Beryllium mirrors can also be used in telescopes.
Atomic properties of beryllium
The atomic mass of an element is determined by the total mass of neutrons and protons that can be found in a single atom belonging to this element. As for the position where to find beryllium within the periodic table of the elements, beryllium is in group 2 and period 2. Beryllium has an atomic mass of 9.0122 u.
The electronic configuration of beryllium is [He] 2s2. The electronic configuration of the elements, determines the form in which the electrons are structured in the atoms of an element. The average radius of beryllium is 112 pm, its atomic radius or Bohr radius is 1113 pm (Bohr radius) pm and its covalent radius is 89 pm.
Beryllium Atomic Number Atomic Mass
You Can Visit Our Managed: Periodic Table Main Page
Beryllium characteristics
Below you can see a table showing the main characteristics of beryllium.
| Beryllium | ||
|---|---|---|
| Chemical symbol | Be | |
| Atomic number | 4 | |
| Group | 2 | |
| Period | 2 | |
| Appearance | white-metallic gray | |
| Block | s | |
| Density | 1848 kg / m3 | |
| Atomic mass | 9.0122 u | |
| Average radius | 112 pm | |
| Atomic radio | 111.3 pm (Bohr Radio) | |
| Covalent radius | 89 pm | |
| Electronic configuration | [He] 2s2 | |
| Oxidation states | 2 (amphoteric) | |
| Crystal structure | hexagonal | |
| State | solid | |
| Melting point | 1551.15 K | |
| Boiling point | 3243.15 K | |
| Heat of fusion | 12.20 kJ / mol | |
| Vapor pressure | 4180 Pa | |
| Electronegativity | 1.57 (Pauling) 1.5 (Allred and Rochow) | |
| Specific heat | 1825 J / (Kkg) | |
| Electric conductivity | 31.35 × 106S / m | |
| Thermal conductivity | 201 W / (Km) | |
Beryllium Discovery Date
You Can Visit Our Managed: Periodic Table Main Page
