The atomic mass of any substance is always equal to 1 mole of that substance The atomic mass added up with itself by 6.02 x 10 23 equals the amount of atoms The atomic mass is the amount of protons plus number of atoms. In honor of Avogadro's contributions to molecular theory, the number of molecules per mole of substance is named the 'Avogadro constant', N A. It is exactly 6.022 140 76 × 10 23 mol −1. The Avogadro constant is used to compute the results of chemical reactions.
The Avogadro constant (symbols: L, NA) is the number of particles (usually atoms or molecules) in one mole of a given substance. Its value is equal to 6.02214129(27)×1023 mol−1. It is the number of units in one mole of any substance. The mole allows scientists to calculate the number of elementary entities (usually atoms or molecules) in a certain mass of a given substance. The constant was named after the Italian scientist Amedeo Avogadro. Avogadro is most famous for his hypothesis that equal volumes of different gases at the same temperature and pressure contain the same number of particles. With Avogadro’s number, scientists can discuss and compare very large numbers, which is useful because substances in everyday quantities contain very large numbers of atoms and molecules.
The measurement of Avogadro’s constant was refined in 2011 to 6.02214078×1023 ± 0.00000018×1023. The mole is the unit for the amount of substance. The number of particles in a substance can be found using the Avogadro constant.

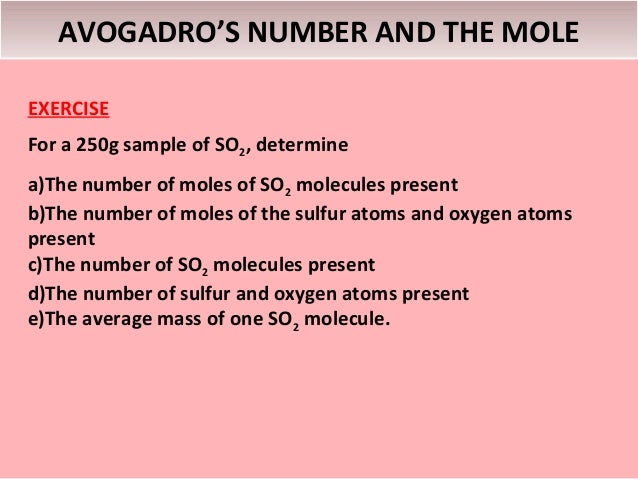
An old term closely related to the Avogadro constant is Avogadro’s number. Avogadro’s number is the number of atoms in 12 grams of the carbon isotope carbon-12. The mass of one mole of a substance is equal to that substance’s molecular weight. Avogadro’s number is a dimensionless quantity and has the numerical value of the Avogadro constant given in base units. For example, the mean molecular weight of water is 18.015 atomic mass units (amu), so one mole of water weight 18.015 grams.
1 Mole Number Avogadro
Example
Calculate the number of water molecules in 0.5 mol of water. Plini guitarist.
Number of water molecules = 6.022 × 1023 × 0.5 = 3.011 × 1023
What Is Avogadro Number
1 Avogadro Number Definition
It is important to be clear about the particles involved. For example, 3.011 × 1023 water molecules contain 9.033 × 1023atoms. This is because a water molecule, H2O, contains three atoms.
